Page Contents
What is Ozone and where it is present?
Ozone (O3) is a highly reactive gas composed of three oxygen atoms. It is formed naturally as well as artificially. Ozone affects life on Earth in good and bad ways depending on where it is in the atmosphere. There are mainly two types of ozone- tropospheric ozone and stratospheric ozone.
Tropospheric or ground-level ozone – what we breathe – is formed primarily from photochemical reactions between two major classes of air pollutants, volatile organic compounds (VOC) and nitrogen oxides (NOx). At lower levels, it is an important greenhouse gas and air pollutant, which is harmful to human and ecosystem health. It is also a major component of urban smog.
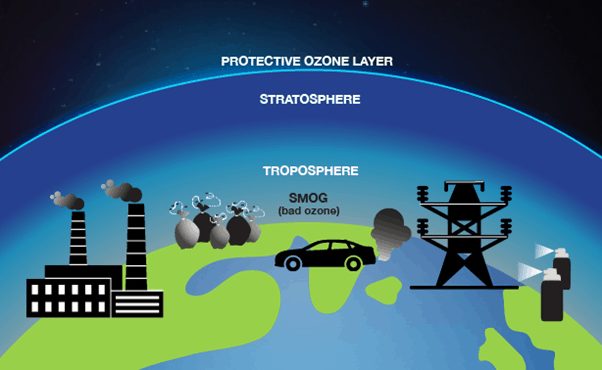
In contrast, stratospheric ozone is formed naturally in the layer of stratosphere through the interaction of solar ultraviolet (UV) radiation with molecular oxygen (O2). The “ozone layer,” where the concentration of ozone is high enough reduces the amount of harmful UV radiation reaching the Earth’s surface.
What is ‘ozone layer’?
The Earth’s atmosphere is composed of several layers. They are troposphere, stratosphere, mesosphere, thermosphere and exosphere. The second layer is called the stratosphere as we go up. It extends from about 10km to 50km.
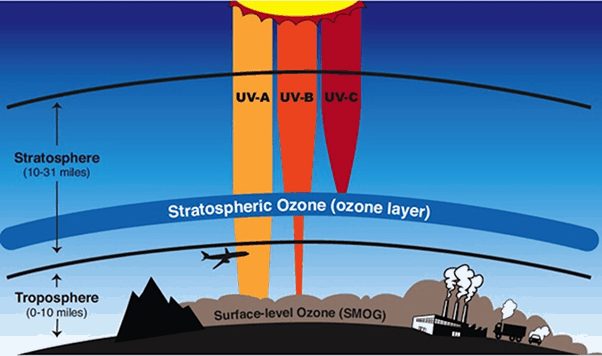
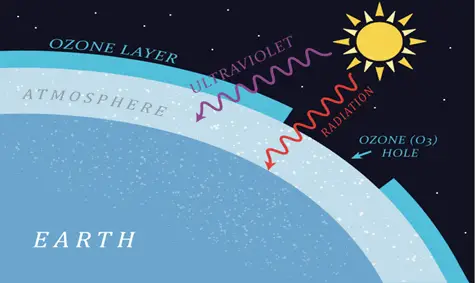
The stratosphere gets warmer at higher altitudes. When UV light is absorbed by oxygen and ozone, heat is generated, which is why the stratosphere gets warmer with altitude. Warm air remains in the upper stratosphere and cool air remains lower, so there is less vertical mixing in this region than in the troposphere. Ozone layer is very important for the earth and it covers the entire planet and protects life on earth by absorbing harmful ultraviolet-B (UV-B) radiation from the sun.
Ozone Depletion
The loss of ozone high in the atmosphere as a consequence of human activities is a serious global-scale environmental problem. For nearly a billion years Earth’s ozone layer has been protecting life on the planet. However, over the past half century, people have unintentionally placed the ozone layer in great danger by polluting the atmosphere.
The most significant of the polluting chemicals are chlorofluorocarbons (CFCs). They are versatile compounds that are chemically stable, odorless, nontoxic, noncorrosive and inexpensive to produce.
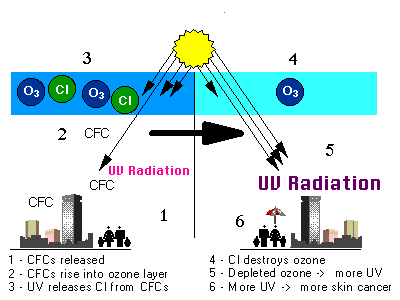
CFCs are used as coolants for air-conditioning and refrigeration equipment, as cleaning solvents for electronic components, as propellants for aerosol sprays, and in the production of certain plastic foams. Global production of CFCs and other ozone depletion substances (ODS) continued to grow rapidly as new uses were found for different kinds of applications. When chlorine and bromine atoms react with ozone in the stratosphere, they destroy ozone molecules. One chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. Ozone can be destroyed more quickly than it is naturally created. Some natural processes like volcanic eruptions also can have an indirect effect on ozone levels. Volcanoes give out large quantities of tiny particles called aerosols. These aerosols in the stratosphere can act as the surface on which chlorine can destroy ozone.
Antarctic Ozone Hole
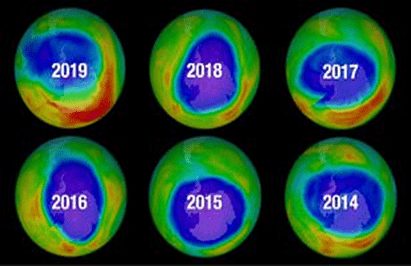
Measurements have shown that ozone concentrations take an especially sharp drop over Antarctica during the Southern Hemisphere spring (September and October). This decreased concentration in the ozone layer is called ‘ozone hole’ and in the early 2000s this ozone hole in the Antarctic region intensified and grew larger until it covered an area roughly the size of North America.
Effects of Ozone depletion
Ozone filters out most of the damaging UV radiation in sunlight. If the concentration of ozone in the ozone layer decreased, these UV rays would reach the surface of the earth and may cause skin cancer and other health issues. The effects of UV radiation on animal and plant life are also important. Crop yields and quality will be adversely affected. Some studies also show that increased UV radiation in the Antarctic will penetrate the waters surrounding the continent and impair or destroy the microscopic plants, called phytoplankton, that represent the base of the food chain.
Read : Climate change
